"江莱生物"产品文献:Self-Strengthened Oxidation-Responsive Bioactivating Prodrug Nanosystem with Sequential and Synergistically Facilitated Drug Release for Treatment of Breast Cancer
Self-Strengthened Oxidation-Responsive Bioactivating Prodrug Nanosystem with Sequential and Synergistically Facilitated Drug Release for Treatment of Breast Cancer
Kaiyuan Wang, Bin Yang, Hao Ye, Xuanbo Zhang, Hang Song, Xia Wang, Na Li, Lin Wei, Yu Wang, Haotian Zhang, Qiming Kan, Zhonggui He, Dun Wang, and Jin Sun
Department of Pharmaceutics, Wuya College of Innovation, School of Pharmacy, Clinical Pharmacy, Wuya College of Innovation, School of Life Science and Biopharmaceutics, and # Key Laboratory of Structure-Based Drug Design & Discovery of Ministry of Education, Shenyang Pharmaceutical University, Shenyang, Liaoning 110016, P. R. China
Key Laboratory of Microbiology, School of Life Science, Heilongjiang University, Harbin 150080, P. R. China
【ABSTRACT】
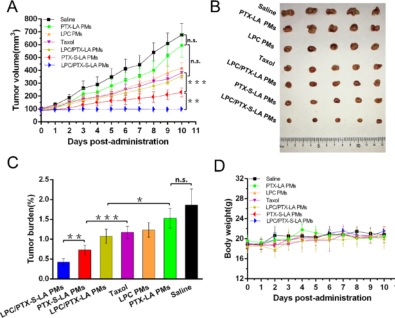
Although environment-sensitive prodrug-based nanoparticles (NPs) have developed rapidly, lots of prodrug NPs still show poor selectivity and efficiency of parent drug bioactivation because of tumor heterogeneity. Herein, self-strengthened bioactivating prodrug-based NPs are fabricated via co-encapsulation of oxidation-responsive thioether-linked linoleic acid-paclitaxel conjugates (PTX-S-LA) and β-lapachone (LPC) into polymeric micelles (PMs). Following cellular uptake, PMs first release LPC to significantly elevate the reactive oxidative species (ROS) level through NAD(P)H: quinone oxidoreductase-1(NQO1) catalysis. Then, NQO1-generated ROS in combination with endogenous high ROS levels in tumor cells could synergistically facilitate PTX-S-LA to release the active cytotoxic agent PTX. Such a novel prodrug nanosystem exhibits selfstrengthened prodrug bioactivation, ultraselective release, and cytotoxicity between cancer and normal cells, prolonged circulation time, and enhanced tumor accumulation, leading to high antitumor efficiency and superior biosafety. Our findings pave the new way for the rational design of oxidation-responsive prodrug NPs for high-efficacy cancer chemotherapy.
【 EXPERIMENTAL SECTION】
The PEG5k−PDLLA5k block copolymer was bought from DaiGang Biotechnology Co., Ltd., China. β-Lapachone was obtained from Nanjing Sheng Sai Chemical Co., Ltd., China. Paclitaxel was bought from NanJing Jingzhu biotechnology Co., Ltd., China. Glycol, linoleic acid, thiodiglycolic anhydride, 4-dimethylami-nopyridine (DMAP), N-(3-dimethylaminopropyl)-N′-ethylcarbodii-mide hydrochloride (EDCI), 1-hydroxybenzotriazole monohydrate (HOBt) were obtained from Energy Chemical Co., Ltd., China. Hoechst and Coumarin-6 (C-6) were bought from Sigma-Aldrich, USA. DiR and DCFH-DA were obtained from Dalian Meilun Biotechnology Co., Ltd., China. The NQO1 ELISA kit was purchased from Shanghai Jianglai industrial Limited By Share Ltd., China[1].
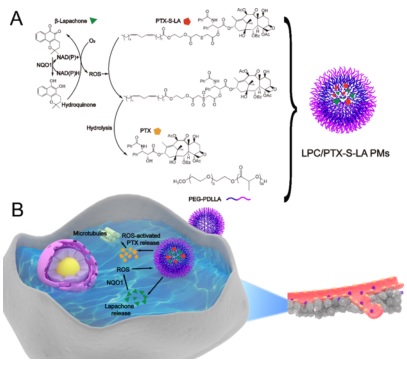
Scheme 1. Schematic Representation of an Oxidation-Responsive Prodrug-Based Nanosystem with Sequential and Synergistically Facilitated Drug Release
a (A) Structure of PTX-S-LA, β-Lapachone and PEG−PDLLA. (B) Prodrug-based nanosystem would accumulate in cancer owing to the EPR effect; following cellular uptake, nanosystem release LPC first, remarkably raising the oxidative stress level via the catalysis of tumor-overexpressing NQO1, inducing a sequential and synergistically facilitated release of PTX from PTX-S-LA.
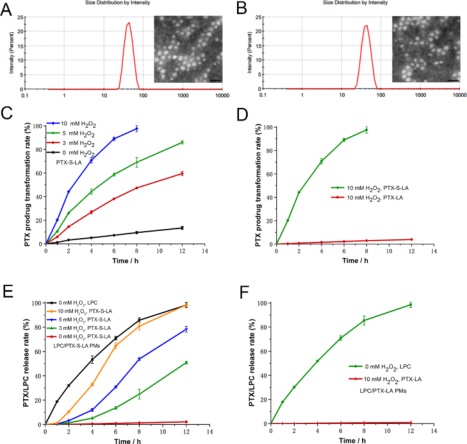
Figure 1. Size and morphology of LPC/PTX-S-LA PMs (A) and LPC/PTX-LA PMs (B); the scale bar represents 100 nm. Transformation of PTX-S-LA (C) and PTX-LA (D) to PTX when incubated with H 2 O 2 . Drug release kinetics of LPC and PTX from LPC/PTX-S-LA PMs (E) and LPC/PTX-LA PMs (F) in PBS complemented with 30% ethanol under H 2 O 2
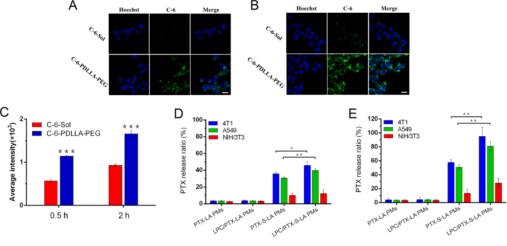
Figure 2. CLSM images of 4T1 cancer cells after incubation with C-6-Sol and C-6-PDLLA−PEG at 0.5 h (A) and 2 h (B). Scale bar, 15 μm. (C) Quantification using flow cytometry for cell uptake in 4T1 cancer cells. Differences from C-6-Sol, ***P < 0.001. PTX release from PMs when incubated in 4T1, A549, and NIH 3T3 cells for 6 h (D) and 24 h (E). (**P < 0.01, *P < 0.05, n = 3).
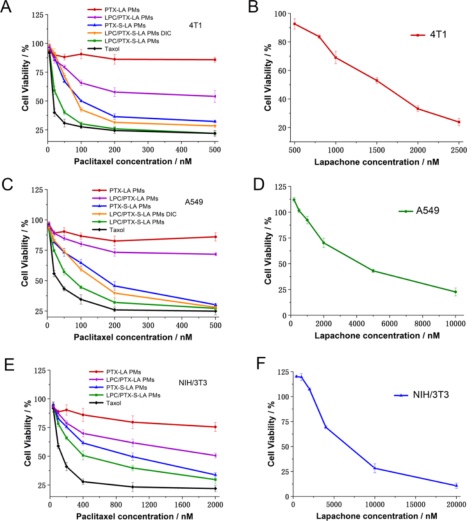
Figure 3. Cell viability of PTX-LA PMs, LPC/PTX-LA PMs, PTX-S-LA PMs, Taxol, and LPC/PTX-S-LA PMs with or without dicoumarol (DIC, a competitive inhibitor of NQO1) against 4T1 (A), A549 (C), and NIH 3T3 cells (E) at different concentrations after 48 h incubation. In vitro cytotoxicity of LPC against 4T1 (B), A549 (D), and NIH 3T3 cells (F) for 48 h.
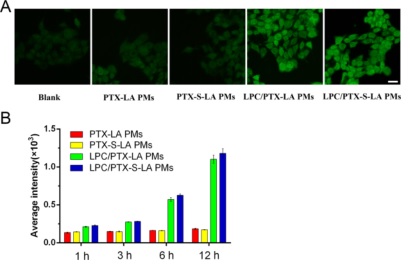
Figure 4. (A) Fluorescence microscopy images of 4T1 cancer cells staining with DCFH-DA after administration with PTX-LA PMs, PTX-S-LA PMs, LPC/PTX-LA PMs, and LPC/PTX-S-LA PMs for 3 h. Scale bar, 20 μm. (B) Cellular ROS generation of 4T1 cancer cells administrated with formulations for 1, 3, 6, and 12 h.
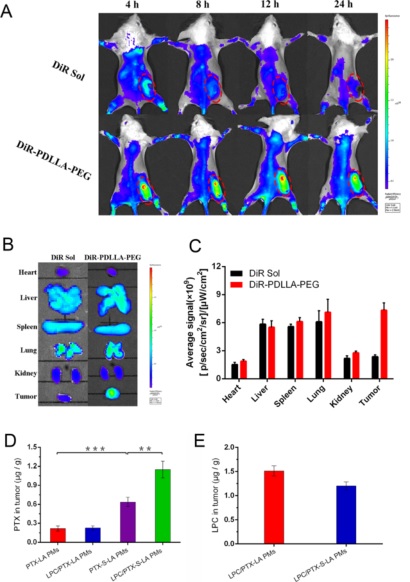
Figure 6. (A) Optical imaging of DiR solution and DiR-PDLLA−PEG PMs (2 mg kg −1 of DiR) for 4, 8, 12, and 24 h. The optical pictures were obtained at an excitation of 748 nm. Fluorescent imaging (B) and quantitative analysis (C) for biodistribution of DiR solution and DiR-PDLLA−PEG PMs (2 mg kg −1 of DiR) in 4T1-bearing BALB/c mice at 24 h (n = 3). Quantification of released free PTX (D) and LPC (E) in tumors at 12 h after administration. (***P < 0.001, **P < 0.01, n = 3).
【REFERENCES】
[1] Chen, Q.; Liu, G.; Liu, S.; Su, H.; Wang, Y.; Li, J.; Luo, C. Remodeling the Tumor Microenvironment with Emerging Nanotherapeutics. Trends Pharmacol. Sci. 2018, 39, 59−74.
[2] Luo, C.; Sun, J.; Sun, B.; Liu, D.; Miao, L.; Goodwin, T. J.; Huang, L.; He, Z. Facile Fabrication of Tumor Redox-Sensitive Nanoassemblies of Small-Molecule Oleate Prodrug as Potent Chemotherapeutic Nanomedicine. Small 2016, 12, 6353−6362.
[3] Sun, B.; Luo, C.; Yu, H.; Zhang, X.; Chen, Q.; Yang, W.; Wang, M.; Kan, Q.; Zhang, H.; Wang, Y.; He, Z.; Sun, J. Disulfide BondDriven Oxidation- and Reduction-Responsive Prodrug Nanoassemblies for Cancer Therapy. Nano Lett. 2018, 18, 3643−3650.
... ...
[备 注] [1]“江莱生物”10年以来一直致力于免疫学技术的积累与沉淀,目前江莱生物实验室占地约3000平米,技术研发团队50余人,配备了全球最先进的分析和检测设备,我们通过自主创新以及与国内外的科研单位合作研发,不断突破新技术,目前我们研发了上千种指标,质量成熟,并长期出口到欧美市场,赢得了众多科研机构的认可 ...
[文献下载] Self-Strengthened Oxidation-Responsive Bioactivating Prodrug Nanosystem with Sequential and Synergistically Facilitated Drug Release for Treatment of Breast Cancer.pdf